Table of Contents
The Birth of Quantum Mechanics
At the start of the 20th century, classical physics struggled to explain why heated objects emitted light in specific wavelengths rather than in a continuous spectrum. This discrepancy, known as the ultraviolet catastrophe, suggested that existing theories were incomplete. Max Planck addressed this issue by introducing the concept of quantized energy emission, proposing that energy could only be exchanged in discrete amounts called quanta. This breakthrough contradicted classical mechanics, which assumed energy varied continuously. Planck’s constant provided a mathematical basis for this discovery, defining the relationship between a quantum of energy and its frequency. His work resolved the ultraviolet catastrophe and initiated the quantum revolution, influencing later theories such as Einstein’s photoelectric effect and Bohr’s atomic model.
Although initially skeptical of his own findings, Planck’s discovery reshaped modern physics, influencing a wide range of applications. His constant later appeared in Schrödinger’s wave equation, which describes electron behavior in atoms, and Heisenberg’s uncertainty principle, which defines the limits of measurement in quantum systems. Planck’s constant also plays a crucial role in technologies like semiconductors, lasers, and atomic clocks, all of which rely on quantum mechanics. The refinement of Planck’s ideas led to new fields, including quantum electrodynamics and quantum computing, which continue to drive scientific advancements. Today, his work remains foundational in physics, proving that quantization governs the microscopic world and defining the very structure of energy interactions.
Planck’s Constant: The Fundamental Quantity
Planck’s constant defines the scale at which quantum effects become significant, establishing a bridge between classical and quantum physics. With a precise value of 6.626 × 10⁻³⁴ Js, it determines the minimum possible energy that can be exchanged at the atomic and subatomic levels. Initially, Max Planck introduced it in 1900 as a mathematical tool to match experimental data on blackbody radiation, but it soon became a cornerstone of quantum mechanics. Without Planck’s constant, equations such as E = hν (which describes the energy of photons) and Schrödinger’s wave equation would lack their defining parameters. Its discovery not only resolved the ultraviolet catastrophe but also paved the way for understanding atomic transitions, spectral lines, and the nature of light itself.
Beyond theoretical physics, Planck’s constant has practical applications in modern technology. It is essential in defining the energy levels of electrons within atoms, explaining why chemical reactions occur at specific energy thresholds. Devices such as lasers, semiconductors, and even LED lights operate based on quantum principles governed by this constant. The 2019 redefinition of the kilogram also relied on Planck’s constant, further demonstrating its significance in metrology. Additionally, advancements in quantum computing and quantum cryptography continue to depend on precise applications of quantum mechanics. By defining the smallest unit of energy transfer, Planck’s constant remains crucial to both fundamental physics and technological innovation.
Planck’s Equation: A Mathematical Breakthrough
Once Planck’s constant was introduced, it led directly to the formulation of Planck’s equation, E = hν, which fundamentally altered the understanding of energy. This equation demonstrated that energy is quantized rather than continuous, contradicting classical wave theories of radiation. Planck derived it to solve the ultraviolet catastrophe, a major paradox in physics where classical models incorrectly predicted that blackbody radiation would emit infinite energy at high frequencies. By assuming that energy could only be emitted in discrete packets, he resolved this issue and provided the first mathematical foundation for quantum mechanics. Planck’s equation later played a critical role in Einstein’s explanation of the photoelectric effect, which confirmed that light behaves both as a wave and as a particle.
Beyond theoretical physics, Planck’s equation has numerous real-world applications. It is essential in understanding atomic transitions, as electrons absorb or emit energy in quantized amounts when moving between energy levels. This concept explains the emission spectra of elements, which are used in spectroscopy to identify substances in fields like chemistry and astrophysics. Additionally, technologies such as LED lighting, fiber-optic communication, and medical imaging rely on principles derived from quantum mechanics. Without Planck’s constant, these innovations would not be possible, as the quantization of energy defines how photons interact with matter. Today, Planck’s equation continues to guide advancements in quantum computing, nanotechnology, and other cutting-edge fields.
The Ultraviolet Catastrophe and Planck’s Solution
Before quantum mechanics, classical physics struggled to explain blackbody radiation, leading to a contradiction known as the ultraviolet catastrophe. According to classical wave theory, as an object heated up, it should theoretically emit infinite energy at high frequencies, an impossible scenario that did not match experimental results. This failure suggested a fundamental flaw in classical physics. Max Planck provided the solution by introducing the idea that electromagnetic energy is quantized rather than continuous. By proposing that energy is emitted in discrete packets, or quanta, he resolved the discrepancy. Planck’s constant played a crucial role in this theory, providing a fixed value that defined the smallest possible unit of energy exchange. This breakthrough marked the birth of quantum physics.
The introduction of quantization fundamentally changed scientific understanding and had profound implications for various fields. Planck’s idea explained why blackbody radiation followed a predictable distribution, aligning with experimental data. His work set the stage for further developments, including Einstein’s photoelectric effect, which demonstrated that light itself is quantized into photons. Planck’s constant also became integral in atomic physics, helping explain electron transitions within atoms and the structure of spectral lines. Today, this concept is applied in cutting-edge technologies such as quantum computing, laser systems, and semiconductor devices. Without Planck’s solution, modern physics and technological advancements reliant on quantum principles would not exist.
Einstein and the Photoelectric Effect
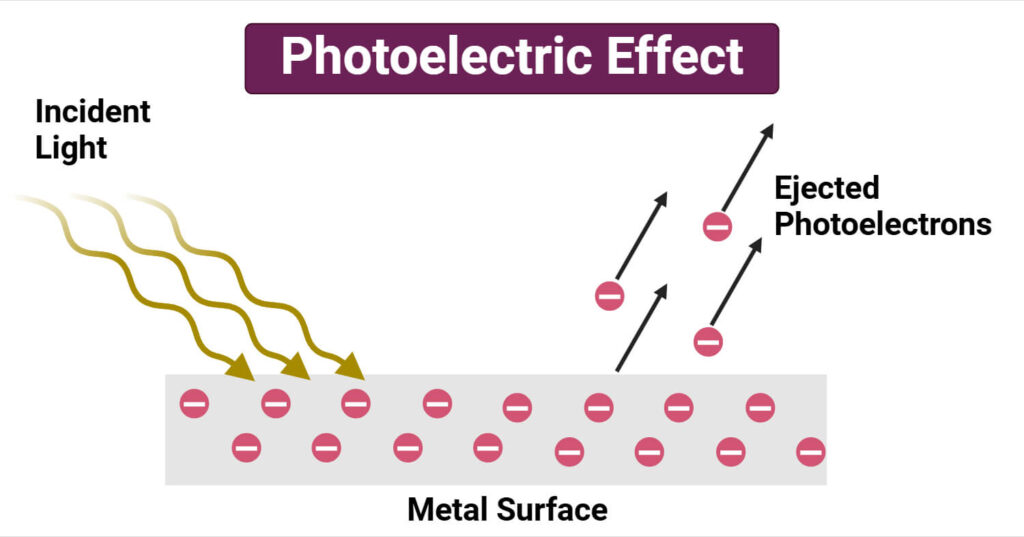
Albert Einstein revolutionized physics in 1905 by applying Planck’s concept of quantized energy to explain the photoelectric effect. Classical physics predicted that increasing the intensity of light should eject more electrons from a metal surface, regardless of frequency, but experiments showed this was not the case. Instead, electrons were only emitted when light exceeded a certain frequency threshold, contradicting the wave theory of light. Einstein proposed that light is made of discrete packets called photons, each carrying energy determined by E = hν. This explanation introduced the concept of wave-particle duality, proving that light behaves both as a wave and a particle. His theory provided direct evidence supporting Planck’s constant, solidifying the role of quantized energy in physics.
Einstein’s discovery had a profound impact on modern physics and technology. The confirmation of the photoelectric effect led to the development of numerous applications, including solar panels, light sensors, and imaging technologies. It also influenced quantum mechanics, shaping the foundation for further breakthroughs in atomic and particle physics. Planck’s constant remains a key component in quantum theory, governing interactions between photons and matter. The photoelectric effect is now a fundamental principle in devices like photomultipliers and night vision technology. Einstein’s work not only earned him the 1921 Nobel Prize but also set the stage for quantum electrodynamics and the exploration of subatomic particles, expanding humanity’s understanding of the microscopic world.
Niels Bohr and Quantum Energy Levels
In 1913, Danish physicist Niels Bohr revolutionized atomic theory by integrating quantum principles into his model of the atom. Classical physics could not explain why electrons did not spiral into the nucleus or why atoms emitted only specific spectral lines. Bohr solved this by proposing that electrons occupy discrete energy levels and can only transition between them by absorbing or emitting a photon of energy E = hν. This concept introduced quantization into atomic physics, demonstrating that energy changes occur in set increments rather than continuously. By incorporating Planck’s constant, Bohr’s model successfully explained the spectral lines of hydrogen, providing the first quantum-based explanation for atomic structure and laying the groundwork for modern quantum mechanics.
Although Bohr’s model was groundbreaking, it was later refined with the development of quantum mechanics. His theory correctly predicted electron behavior in hydrogen but struggled with multi-electron atoms. The advent of Schrödinger’s wave equation and Heisenberg’s uncertainty principle introduced a more accurate depiction of electron motion using probability distributions rather than fixed orbits. Despite these advancements, Bohr’s reliance on Planck’s constant remains fundamental, as it established the concept of quantized energy states. Today, this principle is essential in understanding atomic interactions, leading to applications in lasers, semiconductors, and quantum computing. Bohr’s contributions remain a cornerstone of quantum physics, shaping modern scientific and technological advancements.
The Development of Quantum Mechanics
The discovery of quantized energy by Max Planck set the stage for one of the most transformative scientific revolutions of the 20th century: quantum mechanics. Classical physics struggled to explain atomic behavior, but Planck’s findings led physicists to explore new models of reality. Werner Heisenberg introduced matrix mechanics, which described quantum states using mathematical matrices, while Erwin Schrödinger formulated his famous wave equation. Schrödinger’s equation incorporated Planck’s constant to describe the probabilistic nature of electron motion, replacing Bohr’s fixed orbits with wave functions. These discoveries revolutionized atomic and molecular physics, allowing scientists to predict chemical bonding and reaction mechanisms. The new quantum framework not only explained atomic structure but also introduced concepts like superposition and quantum tunneling.
As quantum mechanics developed, additional breakthroughs expanded its reach into advanced fields of physics and technology. Paul Dirac’s work bridged quantum mechanics and special relativity, leading to predictions of antimatter. Quantum field theory emerged, describing particle interactions in terms of quantized fields, forming the foundation for the Standard Model of particle physics. The implications of Planck’s constant extended beyond theory, influencing semiconductor development, the creation of lasers, and advancements in quantum computing. Today, quantum mechanics underpins much of modern technology, including transistors, MRI machines, and encryption methods. Ongoing research continues to refine quantum theories, with applications ranging from quantum teleportation to the search for a unified theory of physics.
Planck’s Constant in Modern Technology
Planck’s constant plays a fundamental role in modern technology, particularly in devices that rely on precise energy transitions. Spectroscopy, for instance, uses quantum principles to analyze materials by measuring the energy of emitted or absorbed photons, allowing for applications in chemistry, medicine, and astrophysics. Telecommunications also depend on quantum mechanics, as fiber-optic communication relies on the behavior of photons traveling through optical fibers. Semiconductor devices, including transistors and microchips, are designed based on quantum principles, ensuring efficient electronic circuits. Planck’s constant directly influences these technologies by defining the quantized energy levels of electrons, which govern how materials emit and absorb light. Without Planck’s constant, advancements such as LEDs, laser technology, and solar energy harvesting would not be possible.
Beyond consumer electronics, Planck’s constant enables critical innovations in cutting-edge scientific applications. Atomic clocks, which provide the most precise time measurements, rely on quantum transitions within cesium or rubidium atoms, ensuring synchronization for GPS and scientific research. Superconductors, materials that conduct electricity with zero resistance, operate due to quantum effects governed by Planck’s constant, leading to advancements in magnetic levitation and medical imaging, such as MRI machines. Quantum mechanics also drives research in quantum computing, where qubits utilize superposition and entanglement to perform calculations beyond classical computers. As technology continues to evolve, Planck’s constant remains at the core of innovation, influencing developments in energy efficiency, encryption, and next-generation computing systems.
Experimental Verification and Refinement
Since its introduction in 1900, Planck’s constant has been rigorously tested through various experiments, ensuring its accuracy and consistency in physics. Early experiments on blackbody radiation confirmed the quantization of energy, proving that electromagnetic waves emit energy in discrete packets rather than continuously. Later, studies in photoelectric emission and electron diffraction provided further evidence of quantized energy interactions. Precision measurements became increasingly sophisticated with the advent of atomic clocks, which use quantum transitions in cesium and rubidium atoms to maintain precise timekeeping. These experiments relied on Planck’s constant to define the energy levels of atoms, reinforcing its significance. The continued refinement of experimental techniques has made it one of the most precisely measured constants in modern physics.
One of the most significant applications of Planck’s constant in metrology was its role in redefining the kilogram in 2019. Previously based on a physical artifact, the kilogram is now defined using the Planck relation through the Kibble balance, an advanced instrument that measures mass in terms of electrical power and quantum mechanics. This shift eliminated reliance on physical objects, ensuring greater accuracy and stability in fundamental measurements. Advances in quantum optics and high-energy physics also contribute to refining Planck’s constant, particularly in experiments involving photon momentum and laser interferometry. As research continues, new methodologies in quantum mechanics and fundamental physics will further solidify our understanding of this essential constant and its implications.

Challenges and Controversies
When Max Planck introduced the concept of energy quantization, it contradicted the long-standing classical view that energy varied continuously. Many physicists were skeptical, as classical mechanics had successfully explained most physical phenomena up to that point. Even Planck himself initially considered quantization a mathematical convenience rather than a fundamental law of nature. The idea that energy could only be exchanged in discrete packets clashed with conventional wave theories of light. However, experimental validation, particularly through Einstein’s explanation of the photoelectric effect, demonstrated that Planck’s constant accurately described energy interactions. As quantum mechanics developed, it became clear that quantization was not just a theoretical abstraction but a fundamental principle governing the behavior of particles on microscopic scales.
Despite its widespread acceptance today, quantum mechanics still raises profound philosophical and scientific questions. One of the most debated topics is wave-particle duality, where particles like electrons and photons exhibit both wave-like and particle-like behavior depending on the experiment. Additionally, quantum entanglement, where two particles remain correlated regardless of distance, challenges classical notions of locality and causality. These phenomena, rooted in equations involving Planck’s constant, continue to puzzle physicists and fuel discussions about the true nature of reality. The interpretations of quantum mechanics, such as the Copenhagen interpretation and many-worlds theory, remain controversial, highlighting how Planck’s work continues to inspire debate and exploration in modern physics.
Future Implications and Quantum Mechanics
The impact of quantum mechanics extends far beyond its initial discoveries, with emerging technologies heavily relying on its principles. Quantum computing, for example, utilizes the unique properties of superposition and entanglement to perform complex calculations exponentially faster than classical computers. By harnessing the precise energy interactions governed by Planck’s constant, quantum computers process and store information in qubits rather than traditional bits, opening the door to breakthroughs in fields such as materials science, artificial intelligence, and pharmaceutical research. Similarly, quantum cryptography ensures ultra-secure communication by utilizing quantum key distribution (QKD), which prevents eavesdropping by making any interception of data immediately detectable. These advancements highlight the ongoing relevance of Planck’s work in developing next-generation technology.
Beyond technological innovations, quantum mechanics plays a crucial role in addressing some of the most fundamental questions in physics. Scientists continue exploring quantum gravity, aiming to reconcile general relativity with quantum mechanics to create a unified theory of everything. In string theory, researchers use equations involving Planck’s constant to describe the behavior of tiny vibrating strings that may form the fundamental building blocks of the universe. Investigations into dark matter and dark energy also rely on quantum principles to explain the missing mass and energy influencing cosmic expansion. As experimental techniques improve, new discoveries will likely refine our understanding of the quantum world, further cementing Planck’s legacy in shaping the future of science and technology.
Planck’s Legacy in Science and Society
Max Planck’s groundbreaking contributions forever changed the course of physics, establishing quantum mechanics as a fundamental pillar of modern science. His introduction of energy quantization and Planck’s constant provided the framework for understanding atomic interactions, spectral lines, and the behavior of subatomic particles. This discovery resolved long-standing issues in classical physics, such as the ultraviolet catastrophe, and led to the development of technologies like semiconductors, lasers, and nuclear energy. Planck’s equation, E = hν, remains central to quantum mechanics, influencing fields ranging from astrophysics to nanotechnology. His work not only earned him the 1918 Nobel Prize in Physics but also set the stage for later physicists like Einstein, Bohr, and Schrödinger to expand upon his revolutionary ideas.
Beyond scientific achievements, Planck’s legacy extends into modern technology and societal advancements. His discoveries have enabled medical imaging techniques such as MRI and PET scans, which rely on quantum mechanics to provide life-saving diagnoses. GPS systems, fiber-optic communication, and solar panels all function based on principles derived from Planck’s constant, proving its continued relevance. Additionally, his pioneering work has inspired generations of scientists to explore quantum computing, cryptography, and the mysteries of the cosmos. As research pushes the boundaries of physics, Planck’s contributions remain foundational, ensuring that the quantum revolution he initiated over a century ago continues to shape the future of science and technology.
How useful was this post?
Click on a star to rate it!
Average rating / 5. Vote count:
No votes so far! Be the first to rate this post.
Author
-

Meet Dr. Kendall Gregory, a highly accomplished professional with a remarkable academic background and a deep passion for empowering individuals through knowledge. Dr. Gregory’s educational journey began with a Bachelor of Science degree, followed by a Doctor of Chiropractic Medicine, focusing on diagnosing and treating musculoskeletal conditions. He further expanded his expertise with a Master's degree in Oriental Medicine, specializing in acupuncture and Chinese herbology, and a Master's degree in Health Care Administration, emphasizing his dedication to improving healthcare systems. Dr. Gregory combines his extensive knowledge and practical experience to provide comprehensive and integrative healthcare solutions. Through his writings, he aims to inspire individuals to take charge of their health and make informed decisions.
View all posts